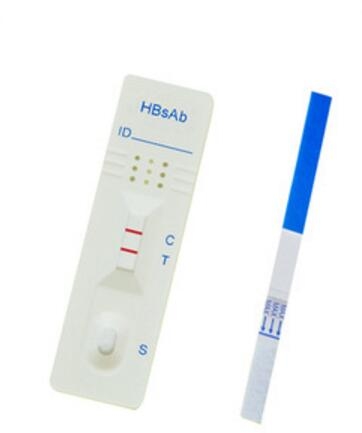
Test Format: Strip/CassetteSpecimen: Whole Blood/Serum/PlasmaExpiry date: 24 MonthsFunction: Rapid testStorage: 4-30 centigrade
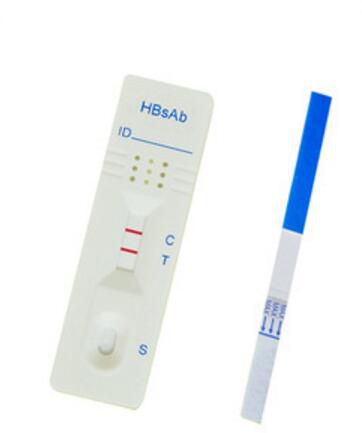
CE Marked IVD Infections disease diagnostic HBsAb rapid test kit Hepatitis B Surface Antibody Rapid test Strip
Catalog of Hepatitis Virus :
| Product Name | Production Information | ||||
| Catalog No. | Specimen Used | Test Format | Kit Size | Sensitivity | |
| HBsAg Hepatitis B Surface Ag Test | H123-11W | Whole blood/Serum/Plasma | Strip | 50 pcs per box | 1mlU/mL |
| HBsAg Hepatitis B Surface Ag Test | H123-23W | Whole blood/Serum/Plasma | Cassette | 25 pcs per box | |
| HBsAb Hepatitis B Surface Ab Test | H124-11W | Whole blood/Serum/Plasma | Strip | 50 pcs per box | 99.60% |
| HBsAb Hepatitis B Surface Ab Test | H124-23W | Whole blood/Serum/Plasma | Cassette | 25 pcs per box | 99.60% |
| HBeAg Hepatitis B Envelope Ag test | H125-11W | Whole blood/Serum/Plasma | Strip | 50 pcs per box | 99.60% |
| HBeAg Hepatitis B Envelope Ag test | H125-23W | Whole blood/Serum/Plasma | Cassette | 25 pcs per box | 99.60% |
| HBeAb Hepatitis B Envelope Ab test | H126-11W | Whole blood/Serum/Plasma | Strip | 50 pcs per box | 91.50% |
| HBeAb Hepatitis B Envelope Ab test | H126-23W | Whole blood/Serum/Plasma | Cassette | 25 pcs per box | 91.50% |
| HBcAb Hepatitis C Core Ab tes | H127-11W | Whole blood/Serum/Plasma | Strip | 50 pcs per box | 93.50% |
| HBcAb Hepatitis C Core Ab tes | H127-23W | Whole blood/Serum/Plasma | Cassette | 25 pcs per box | 93.50% |
Intended Use of HBsAb Rapid Test Device
One Step HBsAb Test is a rapid, immunochromatographic assay for the qalitative detetion of hepatitis B surface antibody (HBsAb) in Human Serum or Plasma. It is intended for use in medical institution as an aid for diagnosis and management of patients related to infection with hepatitis B surface antibody as well for screening of blood donors or blood products
The one step HBsAb test is a double antigen sandwich immunoassay. Colloidal gold conjugated HBsAg complexes are dry-immobilized in the test device. When the sample is added, it migrates by capillary diffusion through the strip rehydrating the gold conjugate complexes. If present, HBsAb will react with the gold conjugate complexes forming particles. These particles will continue to migrate along the strip until the Test Zone (T) where they are captured by HBsAg immobilized there and a visible red line appears. If there is no HBsAb in sample, no red line will appear in the Test Zone (T). The gold conjugate complexes will continue to migrate alone until they are captured in the Control Zone (C) by immobilized goat anti-HBsAg antibody aggregating a red line, which indicates the validity of the test.
Procedure and Result Reading of HBsAb Rapid Test Device
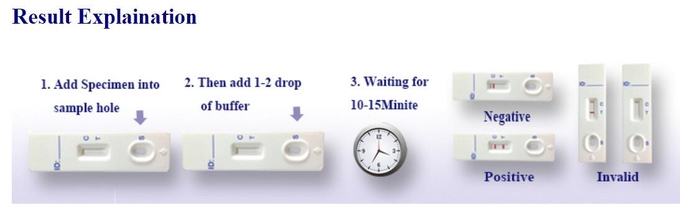
WARNINGS AND PRECAUTIONS
It is recommended that all specimens be handled in accordance with Biosafety Level 2 practices as described in the CDC NIH Publication, Biosafety in Microbiological and Biomedical Laboratories9 or other equivalent guidelines.
1) For in vitro diagnostic use only.
2) Wear gloves to perform this procedure and treat all specimens and used devices as potentially infectious.
3) Clean and disinfect all spills of specimens and reagents using a suitable disinfectant, such as 1% Sodium Hypochlorite.
4) Sterilize all devices used in this assay prior to disposal.
5) Do not use test beyond the expiration date.
6) All positive results must be confirmed by an alternative method.
7) Do not interchange reagents from one kit lot to another.
SAMPLE COLLECTION AND STORAGE
1) Collect serum or plasma specimens following regular clinical laboratory procedures.
2) Storage: A specimen should be refrigerated if not used the same day of collection. Specimens should be frozen if not used within 3 days of collecting. Avoid freezing and thawing the specimens more than 2-3 times before using. 0.1% of Sodium Azide can be added to specimen as a preservative without affecting the results of the assay.
ASSAY PROCEDURE
For serum or plasma specimens:
1) Dispense 2 drops (70 μl) of serum/plasma specimen to the sample pad of the test strip using the plastic dropper provided according to the figure.
2)Start the timer and wait for the colored line(s) to appear.
3) Interpret test results at 15 minutes.




 DE Medical Technology Jiangsu Co.,Ltd
DE Medical Technology Jiangsu Co.,Ltd